Category - Application-note
Performing EPA method 1631 using the PSA Millennium Merlin 10.035 systems
Mercury is naturally present in aquatic systems in very low concentrations. Due to the long range atmospheric transport and deposition of anthropogenic mercury, elevated concentrations of mercury are found even in remote freshwater system although no direct local contamination sources are present. It is long recognised that mercury is one of the most hazardous toxicant to human and the environment. To protect people and the environment from the mercury, governments and regulatory agencies are introducing ever more stringent guidelines. As a result, analysts are challenged to achieve lower detection limits. The use of atomic fluorescence spectrometry for the determination of mercury was first reported by Thompson and Reynolds in 1971. Since then, several authors have described enhancements to the technique that reduced formal instrument detection limits (IDL) for the technique to the 1–10 ng/L range. Knox et al. reported on the use of atomic fluorescence for the determination of mercury with a detection limit of less than 1 ng/L. The USEPA has promulgated Methods 245.7 and 1631 for the determination of low level total mercury in water. The 245.7 method is based on vapor generation atomic fluorescence spectrometry (CV-AFS) without gold amalgamation whereas the 1631 method uses amalgamation to pre-concentrate mercury to provide lower detection limits. Method 245.7 can be performed using the PSA 10.025 or alternatively a PSA 10.035 can be used if the preconcentrator is bypassed. Here we show how the PSA Millennium Merlin system meets the methodology, operation procedure, analytical sensitivity, and the quality control criteria for this method.
Figure 1 PSA 10.035 Millennium Merlin System
The Millennium Merlin system (Figure 1) is designed for routine use, which provides the full range of facilities required by researchers and method development chemists. The Millennium Merlin system offers standard modules that can be configured to meet current legislation. In addition to the EPA Methods 1631 and 245.7 the CV-AFS also complies to European Hg standards EN13506, EN12338 and EN17852.
Figure 2 Schematic flow diagram of PSA 10.025/10.035 Millennium Merlin. Method 245.7 configuration is when pre-concentration is bypassed
Table 1 EPA Method 1631
| Method 1631 | |
| Method of Detection |
Continuous Flow CV-AFS |
| Sample Digestion |
BrCl 0.5% in HCl 0.5% |
| Vapour Generation |
SnCl2 1% in HCl 10% |
| Pre-concentration |
Gold Trap |
| Lowest Calibration Standard |
0.5 ng/L |
| Method Detection Limit (MDL) |
0.2 ng/L |
| Quantification Limit (ML) |
0.5 ng/L |
The USEPA approved the Method 1631 for the determination of low level total mercury in water which specified a detection limit of 0.2 ng/L. With Millennium Merlin system, even better sensitivity (0.05 ng/L) can be achieved. Apart from the sensitivity, Method 1631 also requires to satisfy a formal quality assurance program which comprises the following requirements, at a minimum:
(a) A calibration containing a minimum of 5 non-zero points, with 3 system blanks. The lowest calibration level must be lower than 0.5 ng/L. The RSD of the calibration factors less than 15%. A recovery of 75-125% for the lowest point of the calibration.
(b) Four replicates of initial precision and recovery solution (IPR, 5 ng/L), RSD<21%, recovery of 79-121%
(c) All bubbler blanks must be <0.25 ng/L with a standard deviation (n-1) <0.10 ng/L. All system blanks and must be <0.5 ng/L with a standard deviation (n-1) <0.1 ng/L. All reagent blanks and method blanks must be <0.20 ng/L and <0.50 ng/L, respectively.
(d) Matrix spike in duplicate, with a recovery of 71-125% and a relative percent difference (RPD) < 24.
(e) An ongoing precision and recovery (OPR, 5ng/L) solution must be analysed prior to and after each sample batch, with a recovery of 77-123%.
(f) A quality control sample (QCS) prepared from a different source of Hg standard must be analysed as an independent check of the system performance.
(g) A method detection limit (MDL) <0.2 ng/L.
Figure 1 Method setting of Millennium Merlin system Performing EPA Method 1631
Table 2 Sequence of analysis complying EPA Method 1631 with typical data set
| Type | Name | QC Results | |
| Cal | Blank1 | 3x system blanks | |
| Cal | Blank2 | ||
| Cal | Blank3 | ||
| Cal | 0.5 ppt | CF=25.24704 | Recovery: 115%, pass |
| Cal | 2.5 ppt | CF=24.740925 | - |
| Cal | 5 ppt | CF=25.06386 | - |
| Cal | 10 ppt | CF=24.990604 | - |
| Cal | 20 ppt | CF=25.141035 | RSDCF= 9%, pass |
| Sample | Blanks | CF=25.173262 | < 0.2 ± 0.1 ppt, pass |
| Sample | IPR 5 ppt | 5.20 ± 0.4 ppt | RSD=8%, Recovery=104%, pass |
| Sample | OPR 5 ppt | 4.92 ppt | Recovery=98%, pass |
| Sample | Water | 1.64 ppt | - |
| Sample | Spike 5ppt | 6.78 ppt | Recovery=103%, pass |
| Sample | Spike II 5ppt | 6.23 ppt | Recovery=92%, RPD=8.4, pass |
| Sample | QCS 12.6 ppt | 12.9 ppt | Recovery=102%, pass |
| Sample | Blanks X10 | MDL(3s)=0.15 ppt, pass | |
| Sample | OPR 5 ppt | 4.78 ppt | Recovery=96%, pass |
EPA Method 1631 and 245.7 allows to measure as many as 20 samples in each sample batch between QC samples. In each batch, a number of QC requirements must be met to demonstrate the initial capability of the laboratory. A typical analytical sequence of 1631 is listed in Table 1 which includes a minimum of 3 system blanks, 1 OPR at the beginning and the end of each batch, 4 IPR, 1 QCS and 2 MS/MSD for every ten samples. The system performance is verified if each QC test in the analysis sequence qualifies the criteria mentioned above in the introduction. In addition, a Method Detection Limit shall be determined to be equal or less than 0.2 ng/L. The results of the measurement shall not be reported if any of the criteria fails.
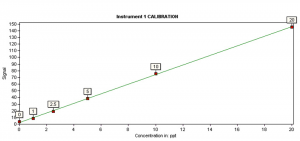
Each sequence also includes a calibration curve with a minimum of five, non-zero standards with the lowest concentration of 0.5 ng/L (5 ng/L for Method 245.7). A typical calibration curve of Method 245.7 is shown in Figure 4, with the lowest calibration standard of 1ppt, which surpasses the EPA requirement.
Method 1631 is a US EPA specified method for the determination ultra low level of total mercury in water samples, with a detection limit of 0.2 ng/L.
The PSA 10.035 Millennium Merlin 1631 not only meets the all the requirements of Method 1631 for measuring mercury in water but even betters the requirements for accuracy and precision, passing all QC tests and achieving an MDL of 0.15 ng/L.
Performing EPA method 245.7 using the PSA Millennium Merlin 10.025 and 10.035 systems
Mercury is naturally present in aquatic systems in very low concentrations. Due to the long range atmospheric transport and deposition of anthropogenic mercury, elevated concentrations of mercury are found even in remote freshwater system although no direct local contamination sources are present. It is long recognised that mercury is one of the most hazardous toxicant to human and the environment. To protect people and the environment from the mercury, governments and regulatory agencies are introducing ever more stringent guidelines. As a result, analysts are challenged to achieve lower detection limits. The use of atomic fluorescence spectrometry for the determination of mercury was first reported by Thompson and Reynolds in 1971. Since then, several authors have described enhancements to the technique that reduced formal instrument detection limits (IDL) for the technique to the 1–10 ng/L range. Knox et al. reported on the use of atomic fluorescence for the determination of mercury with a detection limit of less than 1 ng/L. The USEPA has promulgated Methods 245.7 and 1631 for the determination of low level total mercury in water. The 245.7 method is based on vapor generation atomic fluorescence spectrometry (CV-AFS) without gold amalgamation whereas the 1631 method uses amalgamation to pre-concentrate mercury to provide lower detection limits. Method 245.7 can be performed using the PSA 10.025 or alternatively a PSA 10.035 can be used if the preconcentrator is bypassed. Here we show how the PSA Millennium Merlin system meets the methodology, operation procedure, analytical sensitivity, and the quality control criteria for this method.

Figure 1 PSA 10.025/ 10.035 Millennium Merlin 1631 System
The Millennium Merlin system (Figure 1) is designed for routine use, which provides the full range of facilities required by researchers and method development chemists. The Millennium Merlin system offers standard modules that can be configured to meet current legislations. In addition to the EPA Methods 1631 and 245.7 the CV-AFS also complies to European Hg standards EN13506, EN12338 and EN17852.
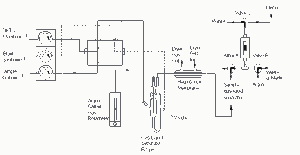
Figure 2 Schematic flow diagram of PSA 10.025/10.035 Millennium Merlin. Method 245.7 configuration is when pre-concentration is bypassed
Table 1 EPA Method 245.7
| Method 245.7 | ||
| Method of Detection |
Continuous Flow CV-AFS |
|
| Sample Digestion |
BrCl 0.5% in HCl 2.5% |
|
| Vapour Generation |
SnCl2 2% in HCl 10% |
|
| Pre-concentration |
None |
|
| Lowest Calibration Standard |
5 ng/L |
|
| Method Detection Limit (MDL) |
1.8 ng/L |
|
| Quantification Limit (ML) |
5 ng/L |
|
Method 245.7 has specified a detection limit of 1.8ng/L. With a Millennium Merlin system, a typical sensitivity of 0.2ng/L can be achieved. The method is shown in Table 1. Method 245.7 is also required to satisfy a formal quality assurance program which comprises the following requirements, at a minimum:
(a) A calibration containing a minimum of 5 non-zero points, with 3 system blanks. The lowest calibration level must be lower than 5ng/L. The RSD of the calibration factors less than 15%. A recovery of 75-125% for the lowest point of the calibration.
(b) Four replicates of initial precision and recovery solution (IPR, 10 ng/L), RSD<16%, recovery of 78-108%.
(c) Matrix spike in duplicate, with a recovery of 63-111% and a relative percent difference (RPD) < 18.
(d) An ongoing precision and recovery (OPR, 10 ng/L) solution must be analysed prior to and after each sample batch, with a recovery of 76-113%.
(e) A quality control sample (QCS) prepared from a different source of Hg standard must be analysed as an independent check of the system performance.
(f) A method detection limit (MDL) < 1.8 ng/L.
Figure 3 Method setting of Millennium Merlin system Performing Method 245.7
Figure 4 Calibration of Millennium Merlin System Performing EPA Method 245.7
EPA Method 245.7 allows the user to measure as many as 20 samples in each sample batch within QC standards. In each batch, a number of QC requirements must be met to demonstrate the initial capability of the laboratory. Each analytical sequence must include a calibration curve with a minimum of five, non-zero standards with the lowest concentration of 5 ng/L for Method 245.7. A typical calibration curve of Method 245.7 is shown in Figure 4, with the lowest calibration standard of 1ppt, which surpasses the EPA requirement.
The PSA 10.035 Millennium Merlin 1631 can easily and quickly be configured to the requirements of EPA Method 245.7 for measuring mercury in water. The instrument is ideally suited for this analysis as it not only meets all the requirements and QC tests for the method but also was better than required for accuracy, precision and method detection limit.
Problems of interferences using Vapour generation Atomic Fluorescence spectrometry Mercury analysers
Vapour generation atomic fluorescence spectrometry offers many advantages in the determination of mercury. Detection limits are in the low ng l-1 range while linearity extends to ?g l-1 levels with good precision. The technique is also relatively free from interferences, which can be split into 2 classes; chemical and spectral. Chemical interferences tend to affect the vapour generation process and usually lead to a suppression of the analytical signal, while spectral interferences during the detection step generally cause a false signal enhancement. Chemical interferences can usually be overcome by selection of an alternative chemistry or by dilution of the sample, an approach made feasible by the inherent sensitivity of atomic fluorescence, while spectral interferences are minimized by the use of vapour generation as a means of sample introduction and the selectivity of the technique.

Quenching
Suppression of the analytical signal due to quenching is caused by collisions between excited atoms and diatomic gases such as O2 and N2 in the atom cell. These collisions cause a deactivation resulting in reduced fluorescence signal. The quenching can be minimized by correct choice of carrier gas to flush the analyte to the detector. Argon is the gas of choice as it has the lowest quenching coefficient. O2 or N2 can be present in samples as dissolved gases in the samples from incomplete digestion or, in the case of the determination of Hg in waters, from the addition of excessive amounts of HONH3Cl for the removal of excess oxidants. In this case Ascorbic acid can be used as an alternative pre-reductant to overcome this issue.
Spectral interferences
The nature of atomic fluorescence as a two step process of absorption of a specific wavelength followed by emission at a particular wavelength means that spectral interferences in atomic fluorescence are very rare. When coupled with vapour generation using tin(II) chloride reduction only Hg is reduced and as such gas phase spectral interference from other volatiles is unlikely especially when good digestions are used. CVAAS in comparison suffers from non specific absorption of aromatics and acid gases such as SO2 and H2S which absorb strongly at 253.7nm.
Chemical interferences
While there are relatively few chemical interferences associated with vapour or hydride generation atomic fluorescence spectrometry, there are certain species which suppress the vapour generation step. However, there are several ways to overcome such interferences, either by modifying instrumental or chemical conditions. Studies were carried out on the interferences caused in the determination of mercury by various species. The data shows that there are very few interferences, the majority of which cause suppression.
There are some interferences which should be considered. Various approaches to overcome or minimize these interferences are outlined below.
Peak shapes can be a very useful diagnostic tool. Continuous flow peaks have very characteristic shapes and if a sample peak does not have this shape it can be indicative of an interferent (although it could also be due to a pumping or gas flow problem). Figure 1 shows a typical peak shape for a mercury standard while figure 2 shows an example of a peak showing significant interference. Figure 2 is actually a peak for a sample which has undergone incomplete digestion. Immediately a peak shape like that in figure 2 is observed the analyst should recognize there is a problem. In this case the problem can be overcome simply by heating the sample digest further and diluting the sample. However, this will not always be the solution, and other approaches may be necessary.
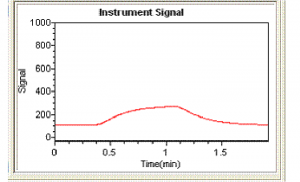
Figure 1 Typical continuous flow peak for mercury
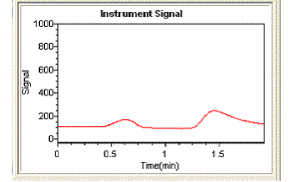
Figure 2 Hg Peak showing severe interference
Sample Dilution
As vapour generation atomic fluorescence offers such low limits of detection, many interferents can be overcome by simply diluting the sample.
Effect of sample preparation
The effect of sample preparation on Hg can be demonstrated by the following . A mercury sample containing various quantities of sodium bisulphide was prepared using HNO3 acid and also by acidification with HCl with Br-/BrO3- oxidation. The nitric acid digestion gave a low recovery because of sulphide interference whereas the bromination digestion oxidizes the sulphide and the interference is overcome by a factor of 10000.
Vapour generation atomic fluorescence for the determination of mercury and the hydride forming elements offers excellent sensitivity, linearity and is relatively free form interferences. Peak shapes are a useful diagnostic tool, allowing identification of potential interference without knowing the concentration in the sample. However, various approaches can be used on the rare occasion where interference is observed. Sample dilution, sample preparation, chemical conditions, alternative reductants or instrumental conditions can all be used to minimize any matrix effects which do occur. In addition to this, standard additions, masking agents, or separation techniques could be used.
Total Mercury Determinations in Drinking, Surface, Ground and Saline Waters
Introduction
Mercury determinations in waters represent a significant part of many routine laboratories’ workloads. The instrumentation and method used must provide quick, accurate and sensitive results. Sample preparation by bromination and detection using the Merlin Plus system fulfil all these requirements and more. Read More